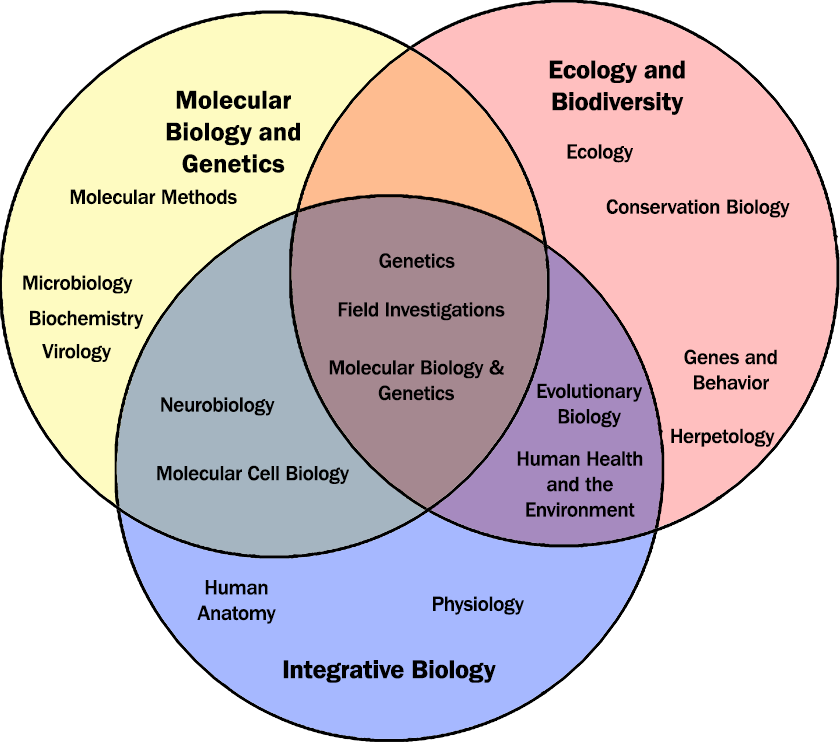
AMPHIBIAN ECOLOGY
Kristen Cecala studies the patterns and processes contributing to changing amphibian distributions in the face of landscape changes including land-use and climate change. She is specifically interested in linking observational and manipulative experiments to explore mechanisms of change. Her research also contributes toward development of comprehensive management strategies for freshwater ecosystems.